Phosphoric Acid (E338) – Side Effects:
Phosphoric acid is a food additive obtained by chemical synthesis, used in the food industry as an antioxidant, acidity regulator, and sequestrant.
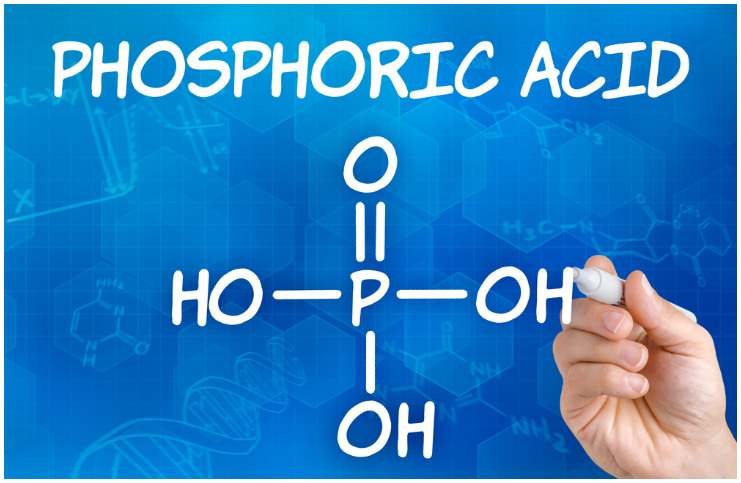
Chemical formula
H3PO4
Molar Mass
97.994 g/mol
Synonyms
orthophosphoric acid, trihydroxylphosphine oxide
Uses (Rust Removal)
In manufacturing, it is a chemical intermediate or reagent in the production of numerous phosphate fertilizers, waxes, agricultural feeds, soaps, polishes, and detergents.
It is added to foods as an acidifying agent, preservative (in flavored waters, cereal bars, processed meats, and bottled coffee beverages), flavor enhancer, and as a clarifying agent.
This compound is also used in processes such as the coagulation of rubber latex, soil stabilization, electropolishing, and as a catalyst for the production of propylene and butene polymers, ethylbenzene, and cumene.
By far, the largest use of E338 comes in the production of fertilizers (>75%). The annual statewide industrial emissions from facilities reporting under the Air Toxics Hot Spots Act in California, based on the most recent inventory, were approximated to be 81,103 pounds of phosphoric acid.
Homeopathy
In homeopathy, it is mainly used in fighting depression.
Hazards & Side Effects of E338 – Phosphoric Acid In Food and Sodas (Coke)
Studies that have been conducted so far are contradictory, but many have found links between the consumption of cola-type soft drinks and kidney disease, kidney stones, or osteoporosis.
It combines very quickly in the digestive tract with calcium and magnesium and thus forms salts that are not absorbed by the body anymore.
In large quantities, it produces hypocalcemia on the bone level and calcification of soft tissues.
It is assumed that one of the causes of osteoporosis is the fact that this substance makes it difficult for the body to absorb calcium and magnesium (it binds to these minerals in the digestive tract, forming compounds that can not be absorbed).
Osteoporosis and related fractures represent major public health problems. The lifetime risk of fracture exceeds 13 percent for men, and 40 percent for women, and hip fractures have been linked with excess mortality of up to 20%.
Soft drink consumption has increased very quickly in the general population in recent years. This behavior has been found to be linked with fractures and low BMD in adolescent girls.
The European Commission asked EFSA (European Food Safety Authority) to review food additives containing phosphorus. This request was generated by a scientific article published in 2012, stating that this type of additive is associated with an increased risk of cardiovascular disease.
A reevaluation must be completed by the 31st of December 2018. Maybe by then, we can say for sure if this additive is harmful or not, and how harmful it is.
Let’s hope that it will be determined whether this additive produces kidney disease, kidney stones (kidney stones can develop in one or both kidneys and most typically affect individuals aged 30 to 60), and osteoporosis. Until then, our recommendation is to avoid foods containing them.
In any case, reducing soda consumption can’t be a bad thing. Not only are you playing it safe with regard to kidney function and osteoporosis (a bone disease that occurs when the body loses too much bone), but you’re also avoiding a lot of extra calories and damage to your teeth.
Sources
It is found naturally in many fruits and vegetables. On an industrial scale, it is produced from phosphate ore.
The acid is a colorless liquid used in fertilizers, detergents, flavorings, and drugs. The most important source of phosphoric acid is soft drinks, particularly cola-type, meat products, and cheeses.
This substance can also be found in toothpaste.
It is a strong chelating agent (combined with metal ions) and is used to treat metal poisonings. Due to this property, it is also used to dissolve rust (iron oxide), which converts into a water-soluble phosphate. Therefore, cola cleans metal objects.
E338 is highly corrosive. Heated acid vapors or in high concentrations (fog) severely attack the respiratory tract.
Concentrated acid solutions attack the skin, causing burns, whereas less concentrated ones cause irritations. Ingestion of concentrated E338 causes upper and lower digestive tract necrosis, pancreas necrosis, and internal bleeding.
Better Alternatives
If you want to lower your intake of phosphoric acid, here are a few healthier alternatives:
Coconut Water
It is the clear liquid found inside a young, green coconut.
It’s packed with amino acids, antioxidants, B-complex vitamins, enzymes, dietary fiber, vitamin C, and minerals, such as – calcium, iron, magnesium, potassium, zinc, and manganese.
Kombucha
This fermented tea drink is booming in popularity due to its powerful health benefits, especially for the gut.
Note – while kombucha is not recommended for people who are sick, this drink does seem to make some healthy people feel even healthier.
Ginger Ale
As the name implies, it contains the herb ginger, which may have medicinal properties. In many countries, people drink it to reduce abdominal pain, nausea, or upset stomach.
Sparkling Bitters
This drink is made by blending sparkling water with highly concentrated liquid extractions of roots, herbs, and fruit
Homemade Almond Milk
It is made by blending almonds with water and then straining the mixture to remove the solids.
The health benefits of almond milk include stronger bones, weight loss, a healthy heart, and improvement of vision.
Green or Black Teas
Both are excellent alternatives to sodas since they come packed with minerals, vitamins, and antioxidants.
Vegetable Juice
You can juice every vegetable you want, be it:
- turnip;
- carrot;
- bitter gourd;
- spinach;
- tomato;
- bottle gourd;
- red cabbage;
- potato;
- cucumber;
- beet.
READ THIS NEXT:
What Are The Side Effects of Aspartame?
References https://pubs.acs.org/doi/abs/10.1021/j150308a009 https://www.ncbi.nlm.nih.gov/pubmed/2198801 https://study.com/academy/lesson/what-is-phosphoric-acid-structure-uses-formula.html